
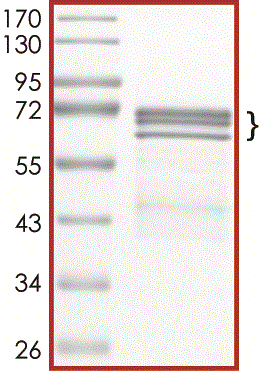
Tau-441, GSK3beta-phosphorylated
Recombinant human tag-free Tau-441 was co-expressed with GSK3beta in E. coli cells. The Tau-441 protein was phosphorylated by GSK3beta in vivo and in vitro prior to the final chromatography purification.
Catalog No. T08-50FN
| Catalog No. | Pack Size | Price (USD) | |
|---|---|---|---|
| T08-50FN-20 | 20 ug | $215 | |
| T08-50FN-50 | 50 ug | $435 | |
| T08-50FN-500 | 500 ug | $1198 | |
| T08-50FN-BULK | BULK | Contact Us |
 Tau-441, BRSK2-phosphorylated, T08-50BN
Tau-441, BRSK2-phosphorylated, T08-50BN

 Toll Free: 1-866-954-6273
Toll Free: 1-866-954-6273 info@signalchem.com
info@signalchem.com