
ERK2, Unactive
Recombinant full-length tag-free human ERK2 was expressed in E. coli cells.
Catalog No. M28-14U
| Catalog No. | Pack Size | Price (USD) | |
|---|---|---|---|
| M28-14U-20 | 20 ug | $215 | |
| M28-14U-50 | 50 ug | $435 | |
| M28-14U-BULK | BULK | Contact Us |

Recombinant full-length tag-free human ERK2 was expressed in E. coli cells.
Catalog No. M28-14U
| Catalog No. | Pack Size | Price (USD) | |
|---|---|---|---|
| M28-14U-20 | 20 ug | $215 | |
| M28-14U-50 | 50 ug | $435 | |
| M28-14U-BULK | BULK | Contact Us |
Overview:
ERK2 is a protein serine/threonine kinase that is a member of the extracellular signal-regulated kinases (ERKs) which are activated in response to numerous growth factors and cytokines (1). Activation of ERK2 requires both tyrosine and threonine phosphorylation that is mediated by MEK. ERK2 is ubiquitously distributed in tissues with the highest expression in heart, brain and spinal cord. Activated ERK2 translocates into the nucleus where it phosphorylates various transcription factors (e.g., Elk-1, c-Myc, c-Jun, c-Fos, and C/EBP beta).
Gene Aliases:
MAPK1, MAPK2, P42MAPK, PRKM1, PRKM2, p41mapk, ERK, ERT1
Genbank Number:
References:
1. Boulton, TG. et al: Purification and properties of extracellular signal-regulated kinase 1, an insulin-stimulated microtubule-associated protein 2 kinase. Biochemistry. 1991 Jan 8;30(1):278-86.
Purity:
Sample Purity Data. For specific information on a given lot, see related technical data sheet.
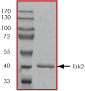
|
Storage, Stability and Shipping:
Store product at –70oC. For optimal storage, aliquot target into smaller quantities after centrifugation and store at recommended temperature. For most favorable performance, avoid repeated handling and multiple freeze/thaw cycles.
Molecular Weight:
~42 kDa
![]() AS Banks et al., An ERK/Cdk5 axis controls the diabetogenic actions of PPAR[gamma] Nature January 2015 10.1038/nature13887
AS Banks et al., An ERK/Cdk5 axis controls the diabetogenic actions of PPAR[gamma] Nature January 2015 10.1038/nature13887
![]() Yang Weiwei et al., ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect Nature Cell Biology November 2012 10.1038/ncb2629
Yang Weiwei et al., ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect Nature Cell Biology November 2012 10.1038/ncb2629
![]() Selvaraja Nagarathinam et al., Extracellular Signal-Regulated Kinase Signaling Regulates the Opposing Roles of JUN Family Transcription Factors at ETS/AP-1 Sites and in Cell Migration Molecular and Cellular Biology January 2015 10.1128/MCB.00982-14
Selvaraja Nagarathinam et al., Extracellular Signal-Regulated Kinase Signaling Regulates the Opposing Roles of JUN Family Transcription Factors at ETS/AP-1 Sites and in Cell Migration Molecular and Cellular Biology January 2015 10.1128/MCB.00982-14
![]() I. Dobrikov Mikhail et al., Dynamic Regulation of the Translation Initiation Helicase Complex by Mitogenic Signal Transduction to Eukaryotic Translation Initiation Factor 4G Molecular and Cellular Biology March 2013 10.1128/MCB.01441-12
I. Dobrikov Mikhail et al., Dynamic Regulation of the Translation Initiation Helicase Complex by Mitogenic Signal Transduction to Eukaryotic Translation Initiation Factor 4G Molecular and Cellular Biology March 2013 10.1128/MCB.01441-12
![]() E. Franklin Norah et al., Differential phosphorylation of the phosphoinositide 3-phosphatase MTMR2 regulates its association with early endosomal subtypes Journal of Cell Science February 2013 10.1242/jcs.113928
E. Franklin Norah et al., Differential phosphorylation of the phosphoinositide 3-phosphatase MTMR2 regulates its association with early endosomal subtypes Journal of Cell Science February 2013 10.1242/jcs.113928
![]() Y. Caro-Gonzalez2 Hector et al., Mitogen-activated protein kinase (MAPK/ERK) regulates adenomatous polyposis coli during growth-factor-induced cell extension Journal of Cell Science March 2012 10.1242/jcs.095166
Y. Caro-Gonzalez2 Hector et al., Mitogen-activated protein kinase (MAPK/ERK) regulates adenomatous polyposis coli during growth-factor-induced cell extension Journal of Cell Science March 2012 10.1242/jcs.095166
![]() Yanga Shuping et al., Phosphorylation of KIBRA by the extracellular signal-regulated kinase (ERK)?ribosomal S6 kinase (RSK) cascade modulates cell proliferation and migration Cellular Signaling February 2014 10.1016/j.cellsig.2013.11.012
Yanga Shuping et al., Phosphorylation of KIBRA by the extracellular signal-regulated kinase (ERK)?ribosomal S6 kinase (RSK) cascade modulates cell proliferation and migration Cellular Signaling February 2014 10.1016/j.cellsig.2013.11.012
![]() Neise Denise et al., Evidence for a differential modulation of p53-phosphorylating kinases by the cyclin-dependent kinase inhibitor p21WAFI/C1P1 Cell Cycle September 2010 10.4161/cc.9.17.12799
Neise Denise et al., Evidence for a differential modulation of p53-phosphorylating kinases by the cyclin-dependent kinase inhibitor p21WAFI/C1P1 Cell Cycle September 2010 10.4161/cc.9.17.12799
![]() Lei Guoa Ming et al., Interactions and phosphorylation of postsynaptic density 93 (PSD-93) by extracellular signal-regulated kinase (ERK) Brain Research July 2012 10.1016/j.brainres.2012.05.026
Lei Guoa Ming et al., Interactions and phosphorylation of postsynaptic density 93 (PSD-93) by extracellular signal-regulated kinase (ERK) Brain Research July 2012 10.1016/j.brainres.2012.05.026
![]() T Honda et al., Phosphorylation of ERK5 on Thr732 is associated with ERK5 nuclear localization and ERK5-dependent transcription. PLoS One February 2015 10.1371/journal.pone.0117914
T Honda et al., Phosphorylation of ERK5 on Thr732 is associated with ERK5 nuclear localization and ERK5-dependent transcription. PLoS One February 2015 10.1371/journal.pone.0117914
![]() C. Salerno John et al., Endothelial nitric oxide synthase is regulated by ERK phosphorylation at S602 Bioscience Reports July 2014 10.1042/BSR20140015
C. Salerno John et al., Endothelial nitric oxide synthase is regulated by ERK phosphorylation at S602 Bioscience Reports July 2014 10.1042/BSR20140015
![]() Bhandaria Deepali et al., Cyclin-dependent kinase 5 activates guanine nucleotide exchange factor GIV/Girdin to orchestrate migration?proliferation dichotomy PNAS July 2015 10.1073/pnas.1514157112
Bhandaria Deepali et al., Cyclin-dependent kinase 5 activates guanine nucleotide exchange factor GIV/Girdin to orchestrate migration?proliferation dichotomy PNAS July 2015 10.1073/pnas.1514157112
![]() R Pal et al., Inhibition of ERK1/2 Restores GSK3β Activity and Protein Synthesis Levels in a Model of Tuberous Sclerosis Sci Rep June 2017 10.1038/s41598-017-04528-5
R Pal et al., Inhibition of ERK1/2 Restores GSK3β Activity and Protein Synthesis Levels in a Model of Tuberous Sclerosis Sci Rep June 2017 10.1038/s41598-017-04528-5
![]() R Pal et al., Inhibition of ERK1/2 Restores GSK3β Activity and Protein Synthesis Levels in a Model of Tuberous Sclerosis Sci Rep June 2017 10.1038/s41598-017-04528-5
R Pal et al., Inhibition of ERK1/2 Restores GSK3β Activity and Protein Synthesis Levels in a Model of Tuberous Sclerosis Sci Rep June 2017 10.1038/s41598-017-04528-5
![]() K Fujita et al., HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer's disease. Science Reports August 2016 10.1038/srep31895
K Fujita et al., HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer's disease. Science Reports August 2016 10.1038/srep31895
![]() et al., Signal Transduction and Targeted Therapy Signal Transduction and Targeted Therapy March 2023
et al., Signal Transduction and Targeted Therapy Signal Transduction and Targeted Therapy March 2023
Angiogenesis, Apoptosis/Autophagy, Cancer, Cardiovascular Disease, ERK/MAPK Pathway, Invasion/Metastasis, Neurobiology, Ser/Thr Kinases
STAY CONNECTED
Fax: 1-604-232-4601